| Student Outline | Developmental and Physiological Aspects of the Chicken Embryonic Heart |
Download this stock image:. A laboratory manual and text-book of embryology. CHICK EMBRYO OF EIGHTEEN PRIMITIVE SEGMENTS 61 Transverse Section through the Primitive (Hensen's) Node or Knot (Fig. 40).—The section shows the three germ layers bound together at the "knot" or node into a mass of undifferentiated tissue. The mesoderm is split laterally into the somatic. 48-hour Chick Embryo-Sections 2-28. Click on the small images to see a larger version of the image. Small images with purple numbers link to large imageswith labels.Small images with black numbers link to large images without labels. Chicken: 22-28 h. In the stage from 22 hours on, the somites formed in the mesoderm at the left and right side of the neural walls become visible. After 24 hours 4 to 5 segmented paired blocks can be discerned. Later on, these structures will differentiate into the vertebrae, the ribs, a part of the skin and the dorsal muscles.
- Dextro-dorsal view ( X 14) of entire chick embryo of 36 somites (about three days incubation). The cranial and cervical flexures which appeared in embryos during the second day have increased so that in three-day and four-day chicks the long axis of the embryo shows nearly right-angled bends in the mid-brain and in the neck region.
- 48 hr serial - Free download as Powerpoint Presentation (.ppt) or view presentation slides online. Chick Embryo 72 Hours. Collapse section.
Introduction
 The development of the heart involves a series of cellular migrations, fusions, and specific differentiations, i.e., a multitude of morphogenetic mechanisms.
The development of the heart involves a series of cellular migrations, fusions, and specific differentiations, i.e., a multitude of morphogenetic mechanisms. 
| The heart of the chicken embryo develops from the fusion of paired precardiac mesodermal tubes located on either side of the developing foregut, on the ventral surface. Between 25 and 30 hours of incubation, the paired heart vesicles begin to fuse at the anterior (head) end and continue to fuse posteriorly to form one continuous tube. After fusion is complete, the heart tube is ventral to the foregut and has four distinct regions that can be identified from anterior to posterior: conotruncus, ventricle, atrium, and sinus venosus. Blood flows anteriorly, from the sinus venosus to the conotruncus. At approximately 33 hours the heart tube bends to form an 'S' shape, with the prominent ventricle bulging to the right. | Dorsal view of a 33-hour chicken embryo. The conotruncus (ct), ventricle (v), atrium (a), and sinus venosus (sv) are evident within the S-shaped heart. |
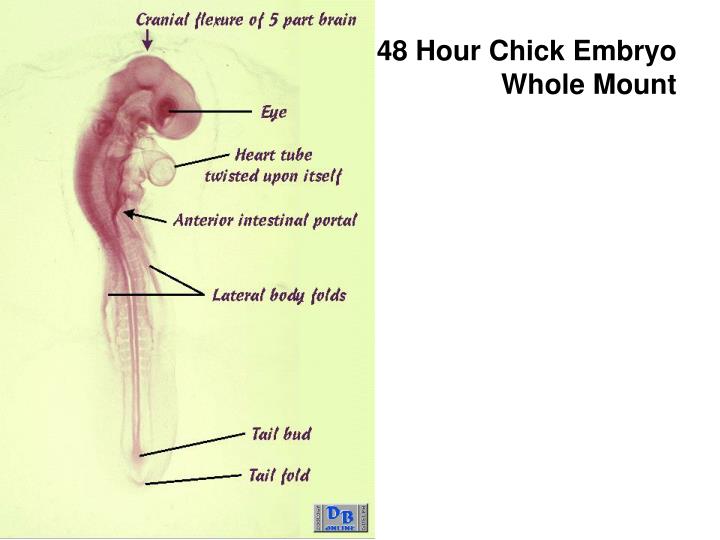
| By 48 hours, the heart has folded upon itself, forming a single loop. This moves the sinus venosus and atrium to a position anterior and dorsal to the ventricle and the conotruncus. The ventricle is U-shaped and in the medial ventral position. Now, the blood flows posteriorly and then makes a sharp turn to flow anteriorly. 56-hour chicken embryo. |
| In the 72-hour embryo, the atrium has begun to expand to the left in preparation of the division into the right and left atria. Although the heart still has two chambers at this time, communication between the sinus venosus and the atrium is via the right side of the atrium. This is the first step towards the sinus venosus becoming part of the future right atrium. The conotruncus will eventually give rise to the aorta. The heart begins to beat just after the paired heart rudiments begin to fuse, immediately before the conotruncus forms. Once the heart tubes have completely fused, the sinus venosus becomes the embryonic pacemaker. Eventually, when the atrium and ventricle each divide into a pair of chambers, and a typical four-chambered heart is present, the sinus venosus is incorporated into the right atrium where it gives rise to the sinoatrial node, the mature pacemaker. | The atrium is now expanded on the right and left sides |
This lab has six goals:
- To identify the anatomy of the developing chicken heart.
- To determine if the heart muscle has an intrinsic ability to contract by surgically removing a beating heart from the living embryo.
- To determine which region of the chicken heart controls the heart beat by isolating the atrium, ventricle, and sinus venosus and then observing which regions continue to beat while in isolation.
- To determine the direction of blood flow through the developing heart.
- To show how in vivo, in vitro, and micromanipulative techniques can be used to study developmental and physiological processes.
- To observe the effects of various concentrations of caffeine and/or gin on an embryonic heart. Your written lab report will be based on this component.
Materials Per Student Pair
48 Hour Chick Embryo Serial Cross Section Pictures
- 72-hour old chicken embryos/eggs, in 37 C humidified incubator (7)
- 110 mm diameter glass dishes, lined with cotton (2)
- Scotch Magic Tape (1)
- 20 G needle attached to a syringe (1)
- Fine scissors (2)
- Fine forceps (2)
- 65 mm Syracuse glass dishes (2)
- Chick saline in 45 C water bath (100 ml)
- Test tube rack in 45 C water bath
- Filter paper 'doughnuts' (8)
- Microknife (Tyler, 1994) and/or iris microdissecting scissors (2)
- Test tubes with caps (6)
- Test tube rack on bench top (1)
- 1.5 ml dropping pipettes (6)
- Embryo spoon (1)
- Gooseneck lamp with 100 W bulb (2)
- Large beaker for eggs/embryos after they have been examined
- Dissecting microscope with lighting from above (2)
- Labeling tape and indelible ink pen (1)
- Atlas
- Stop watch or clock with second hand
- Heart models
- Stock solution of 3% caffeine, made with chick saline
- Stock solution of gin, made with chick saline
Making a Windowed Egg
..... - Modified from Cruz {1993}
- Pick up an egg from the incubator, maintaining its horizontal position as you carry it back to your bench. Place the egg, in that horizontal position, in the glass dish that is lined with cotton.
- Place Scotch Magic tape along the long axis of the egg, so that it covers most of the 'top' of the egg. Place 2 more pieces of tape on the egg, on either side of the center piece.
- Cover the rounded end of the egg with a small piece of Magic tape.
- Puncture the rounded end of the egg that is covered by the tape with a 20 G needle. Insert the needle into the egg so that the needle is pointing down. Be careful of your fingers!
- Withdraw 1-2 ml of albumen. This allows the embryo (if there is one) to move away from the upper surface of the egg, where you will be cutting out the window. Save the syringe and the albumin.
- CAREFULLY, puncture the taped-covered top of the egg with the tip of your scissors. (Again, be careful of your fingers.) The location of the puncture should be about a half an inch off center.
- Proceed to cut out an oval opening, pulling up with your scissors so that you are keeping them as far away from the embryo and vitelline envelope as possible.
- The size of the opening depends on the size of the egg; it should be about the size of a quarter. With forceps, remove the shell cap, exposing the window.
- If you are going to observe the embryo for more than a couple of minutes while it is still in the egg, you will need to prevent dehydration; add several drops of the chick saline to the surface of the embryo. If you are going to immediately explant the embryo, do not add any saline. It will prevent the filter paper doughnut from adhering to the vitelline envelop.
- Determine the in vivo heart rate, the number of beats per minute. Record this information in Table 1. Then draw and label a picture of the in vivo embryo (Appendix A).
Explantation of a Chicken Embryo
..... - Modified from Cruz {1993}
- Explantation means an embryo or tissue is removed from its normal environment, in this case the egg, and placed in another location, in this case a dish of saline. This is a useful method because it is much easier to manipulate or operate on an embryo when it is in a dish, rather than in an egg.
- With the forceps, gently pick off any lumps of albumen on the egg's surface until it appears almost dry.
- The next step requires you to place a filter paper doughnut on the blastoderm so it frames the embryo. The filter paper will stick to the vitelline envelop, holding the embryo in the center. You will then cut the filter paper off the surface of the egg, lifting the embryo with it. When you finally free the filter paper, the embryo should be framed within the filter paper. It's then transferred to the dish of warm saline.
- With forceps, gently position a filter paper doughnut on top of the vitelline envelope, framing the embryo. Gently pat the doughnut in place with the tips of the forceps. Wait a minute to allow the vitelline envelope to adhere to the filter paper.
- While waiting, fill one of the small dishes with about a quarter of an inch of warm chick saline and place the dish on the stage of your dissecting microscope. Angle the gooseneck lamp so that it come as close as possible to the dish; this keeps the saline warm. This is a critical step since the chicken embryo's normal body temperature is close to 37 C. Why do you think temperature is such a critical factor?
- Hold one edge of the doughnut with the tips of the forceps and cut the vitelline envelope along the edge of the doughnut with a pair of scissors. Slowly work your way around the rim of the doughnut, carefully checking to see that the vitelline envelope adheres to it.
- Gradually lift the doughnut with the forceps - the embryo should remain within the center of the doughnut. Quickly transfer the explant to the small dish with the warmed chick saline. You can keep the embryo 'upright' so the right side is facing up. Or you can flip over the explant so the surface that was facing the yolk (the left side) is now facing upwards.
- If the embryo doesn't stay attached to the filter paper, this is the time to use your embryo spoon. Remember to change your saline solution several times since much yolk will be transferred using this process.
- Place the dish on the stage of the dissecting microscope.
- To keep the embryo alive as long as possible, place the illuminated gooseneck lamp close to the dish; this will keep the embryo's body temperature close to 37 C. You should also periodically add fresh, warm chick saline.
- Draw and label a picture of the in vitro embryo (Appendix A). In your diagram indicate the direction of the blood flow through the heart.
- Determine the in vitro heart rate, the number of beats per minute. Record this information Table 1. How does this heart rate compare with the in vivo heart rate?
Electrophysiology of the Embryonic Heart
72 Hour Chick Embryo Labeled
- The 72-hour old chick explant should be oriented so the right side is facing upwards; this allows you to access the beating heart. Add some fresh, warm chick saline.
- Determine the heart rate, beats per minute, of the in vitro heart. Record this information in Table 1. How does this rate compare to the in vivo heart rate?
- Fill a clean small dish with some warm chick saline, about a quarter of an inch deep.
- Surgically remove the beating heart by cutting it above the conotruncus and below the atrium or sinus venosus.
- Observe the beating of the excised heart and record the following information in your lab notebook and the table at the end of the handout.
- Where does the beating begin and end?
- Determine the heart rate, number of beats per minute and record in Table 1. Is it similar to or different from the heart beat in the explanted, in vitro embryo?
- Draw and label a picture of the explanted heart, labeling each region (Appendix A).
- If your explanted heart stops beating, change the saline and place the dish closer to the gooseneck lamp.
Explanted 72-hour
chicken heart
showing isolated
heart chambers:
sinus venosus (sv),
atrium (a) and
ventricle (v). - Use the microknife or iris microdissecting scissors to isolate each of the two or three regions of the heart. If you can not easily identify the sinus venosus and conotruncus, just cut between the atrium and ventricle.
.....
- Observe each isolated tissue and record the following information in your lab notebook and Table 1.
- Does each have an intrinsic heart beat? If so, are they synchronous?
- What is the heart rate for each region? Do they beat at the same rate as the in vivo or in vitro heart?
- Draw a properly labeled picture of the isolated chambers (Appendix A).
Effects of Caffeine and/or Alcohol on Heart Rate

- Prepare the dilutions of the drug with which you chose to work. Make each dilution in one of the test tubes and use the warm chick saline to create the dilutions. Store your labeled test tubes in the test tube rack that's in the 45 C water bath.
- Design a table similar to the one below wherein you recorded all of your previous data. Remember to include both in vivo and in vitro embryonic heart rates before you expose your embryos to the drugs.
- Explant an embryo into a clean dish of saline. Remember, the temperature of the solution that surrounds the embryo has a significant effect on the heart rate. So, keep the embryo and your solutions at a temperature as close to 37 C as possible.
- Be sure to have a baseline heart rate for each embryo before exposing them to the drug.
- Carry out your experiment!
| Embryo # | In vivo Embryo | In vitro Embryo | Explanted heart | Isolated heart chambers | |
|---|---|---|---|---|---|
| Atrium | Ventricle | ||||
| . | . | . | . | . | . |
| . | . | . | . | . | . |
| . | . | . | . | . | . |
| . | . | . | . | . | . |
| . | . | . | . | . | . |
| . | . | . | . | . | . |
Clean Up
- Put all yolk, albumen, and embryos in the beaker.
- Throw the egg shells and cotton in the trash.
- Put the syringes in the red 'Sharps' receptacles.
- Clean your forceps, scissors and microknife with wet paper towel. Be sure to remove all traces of albumen and yolk. Thoroughly dry them with paper towel.
- Clean your bowls, empty and clean the test tubes, and let them air dry.
- WASH YOUR HANDS WITH SOAP AND WATER!

This page was last modified May 27, 1999. Send questions or comments to jxm57@psu.edu
72 Hour Chick Embryo
Copyright© 1999 Dr. Jacqueline McLaughlin and Dr. Elizabeth R. McCain All Rights Reserved
This material may not be reproduced without expressed written permission from the authors.